Introduction
Essential thrombocythemia (ET) and polycythemia vera (PV) are well described in adolescent and young adults (AYA) patients (pts); the most prevalent complications are thrombotic events, but progression to myelofibrosis (MF) is also associated with significant morbidity and mortality and this risk is time-dependent. Here we investigated the impact of cytoreductive drugs on outcomes in a well-defined cohort of AYA pts.
Method and objectives
Patients with a diagnosis (Dx) of ET or PV established at less than 25 years (yrs) of age and having known driver mutation status were included from a collaborative group of centers within the EHA MPN special working group. Patients were classified by the first cytoreductive treatment they received or no cytoreduction (NoCYTO). Main endpoints were thrombosis free survival (TFS) and, for those who received a minimum of 2 years of treatment, myelofibrosis free survival (MFS). Kaplan-Meier and log-rank tests were used to compare groups.
Results
Overall we included 348 pts (278 ET, 70 PV) with median age 20 yrs at Dx (IQR: 18-23). Median age at Dx for ET was 21 yrs (18-23) and for PV 20 yrs (16-23). 249 pts were female (216 ET, 33 PV). Median follow-up was 9 yrs (4-15). Overall, 237/348 (68%) started a cytoreductive therapy. First treatment was hydroxycarbamide (HU) in 126 pts (100 ET, 26 PV), IFN in 55 pts (33 ET, 22 PV), anagrelide (ANA) in 52 pts (51 ET, 1 PV) and other in 4 pts (1 ET, 3 PV). 111 pts (32 %) did not receive cytoreduction (93 ET, 18 PV) deemed NoCYTO. According to ELN risk group at Dx, 39/43 pts (26 ET, 13 PV) receiving a cytoreductive drug were high-risk and 197/304 pts (159 ET, 38 PV) were low-risk. Reason for treatment initiation was thrombotic event in 32 pts (24 ET, 8 PV), platelets >1000x10 9/L in 96 pts (87 ET, 9 PV), MPN-related symptoms 12 pts (7 ET, 5 PV), and other/unknown for 97 pts (67 ET, 30 PV).
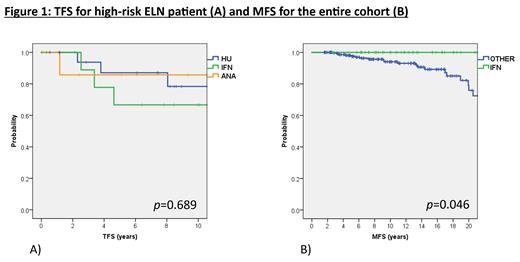
Ten year TFS was 87% (95%CI: 82-91%) overall, 87% (82-92%) for ET and 86% (77-96%) for PV. In multivariate analysis, elevated WBC (>11x10 9/L) was associated with higher thrombosis risk (HR: 2.8, 95%CI: 1.2-6.2, p=0.01) and splenomegaly with a lower risk (HR: 0.2, 95%CI: 0.1-0.9, p=0.04). In the ELN low-risk group, 10yrs TFS was 82% (73-90%) for HU, 84% (70-98%) for IFN, 93% (84-100%) for ANA and 94% (88-100%) for noCYTO, suggesting no benefit for cytoreduction, p=0.04. For high-risk patients, 10yrs TFS was 78% (56-100%) for HU, 67% (35-98%) for IFN, and 86% (59-100%) for ANA, p=0.689 (Fig 1A).
Ten year MFS was 95% (92-98%) overall, 95% (91-98%) for ET, 93% (85-100%) for PV. On multivariate analysis, CALR mutation (HR: 6.0, 95%CI: 2.3-16.1, p<0.05) and interestingly thrombotic history at Dx (HR 3.8, 95%CI: 1.3-11, p=0.02) were associated with increased risk of MF progression overall. For ET: CALR mutation (HR 4.1, 95%CI: 1.6-10.5, p <0.05) and splenomegaly (HR: 2.9, 95% CI: 1.1-7.7, p=0.03) were associated with MF progression, but only CALR mutation was significant on multivariate analysis (HR: 4.2, 95% CI: 1.6-11.6, p<0.05). For PV, we did not identify risk factors associated with MF progression. Finally, we investigated the impact of cytoreductive therapy on MF progression. Overall, 10yrs and 20yrs MFS for IFN was 100%, for HU 10yrs and 20yrs MFS were 93% (86-99%) and 74% (57-92%), for ANA 10yrs and 20yrs MFS were 92% (82-100%) and 73% (40-100%), and for NoCYTO patients 10yrs and 20yrs MFS were 94% (88-100%) and 74% (47-100%), respectively (Fig 1b). Log-rank test comparing IFN vs. other management (HU, ANA or NoCYTO) was significantly in favour of IFN (p=0.046).
Finally, during the follow-up there was only one MDS-progression and four deaths (1 of GVHD following HSCT 34 yrs after Dx, 1 of CMV disease 15 yrs after Dx, 2 of unknown cause, 4 yrs and 41 yrs after Dx).
Discussion
This study is the largest of its kind in contemporary young ET and PV patients focusing on specifically upon the impact of treatment. We demonstrate that early initiation of cytoreduction in low-risk patients does not impact TFS and that the choice of drug does not impact TFS in high-risk patients. In addition, importantly our data demonstrate that IFN specifically compared to other cytoreductive agents yields significantly better MFS compared to other treatments. These results support the use of IFN as a currently available disease-modifying agent to improve long-term MFS and also warrant reconsideration of earlier treatment in patients with ET and PV with IFN to improve MFS potentially as a primary aim.
Disclosures
Devos:AOP Pharma: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; BMS-Celgene: Consultancy, Honoraria; MorphoSys: Consultancy, Honoraria. McMullin:Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AOP: Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CTI: Membership on an entity's Board of Directors or advisory committees; Sierra oncology: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees. Legros:Incyte Biosciences: Honoraria; BMS: Honoraria; NOVARTIS: Honoraria, Other; Correspondances en Hématologie: Consultancy, Honoraria, Speakers Bureau; PFIZER: Honoraria; AMGEN: Honoraria. Zweegman:Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Research Funding. Skoda:BMS/Celgene,AOP, GSK,Baxalta, Pfizer, and Novartis: Consultancy, Honoraria, Speakers Bureau; F. Hoffmann-La Roche: Research Funding; Ajax Therapeutics: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees. Griesshammer:Novartis: Consultancy, Honoraria, Speakers Bureau; AOP Orphan: Consultancy, Honoraria, Speakers Bureau; BMS: Consultancy, Honoraria, Speakers Bureau; AbbVie: Consultancy, Honoraria, Speakers Bureau; Pfizer: Consultancy, Honoraria, Speakers Bureau; Roche: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Gilead: Consultancy, Honoraria, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Speakers Bureau; Sierra: Consultancy, Honoraria, Speakers Bureau. Kiladjian:Incyte Corporation: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; AOP Orphan Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; AbbVie, AOP Health, Bristol-Myers Squibb, GlaxoSmithKline, Incyte, Novartis, Pharmaessentia.: Consultancy. Harrison:CTI: Honoraria, Speakers Bureau; GSK: Honoraria, Speakers Bureau; AOP: Honoraria, Speakers Bureau; Novartis: Honoraria, Research Funding, Speakers Bureau; Abbvie: Honoraria, Speakers Bureau; Morphosys: Honoraria, Speakers Bureau; Galecto: Honoraria, Speakers Bureau; BMS: Honoraria, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal